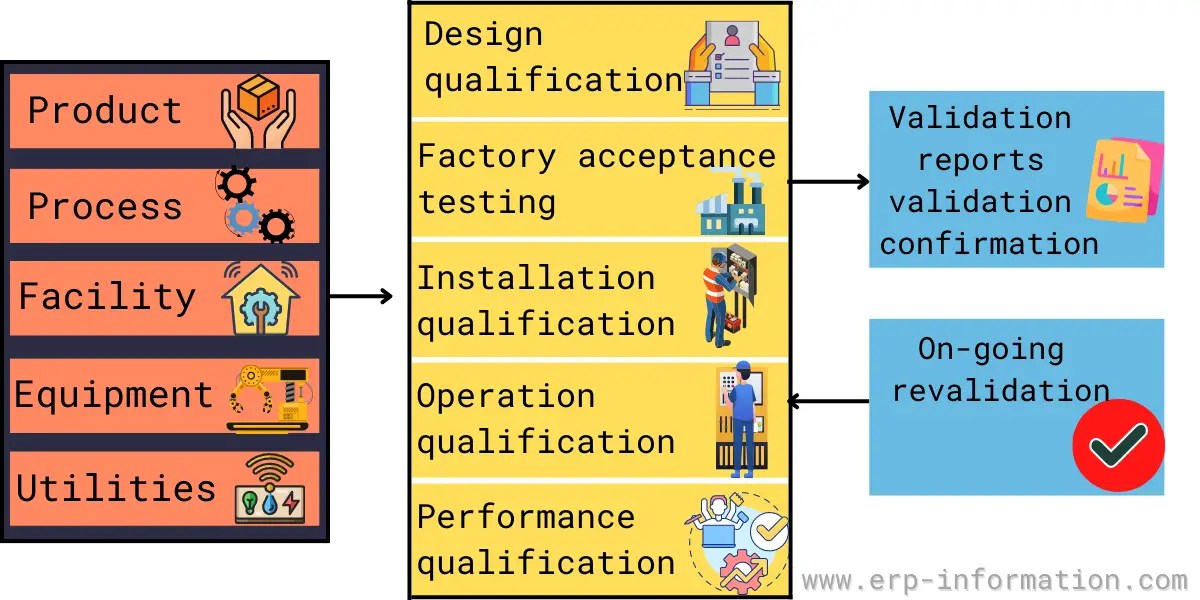
Validation Master Plan Template - Buy a fully editable and compliant document template for recording the schedule and status of validations for medical devices. Learn what the validation master plan (vmp) is and how to prepare for the medical device process validation according to iso 13485. Discover the essential components of a validation master plan in the pharmaceutical and medical device industries, understanding its importance. Learn how to prepare a validation master plan (vmp) for drug product or active pharmaceutical ingredient manufacturing and. You can create a great protocol, using a template. Three (3) options to create a validation master plan.
Validation Master Plan (VMP) Downloadable Interactive Template.
Learn what the validation master plan (vmp) is and how to prepare for the medical device process validation according to iso 13485. Buy a fully editable and compliant document template for recording the schedule and status of validations for medical devices. You can create a great protocol, using a template. Three (3) options to create a validation master plan. Learn.
What is Validation Master Plan? (Template, Examples)
Learn what the validation master plan (vmp) is and how to prepare for the medical device process validation according to iso 13485. Discover the essential components of a validation master plan in the pharmaceutical and medical device industries, understanding its importance. Three (3) options to create a validation master plan. Buy a fully editable and compliant document template for recording.
FREE 9+ Sample Validation Plan Templates in PDF MS Word
Discover the essential components of a validation master plan in the pharmaceutical and medical device industries, understanding its importance. Three (3) options to create a validation master plan. You can create a great protocol, using a template. Learn what the validation master plan (vmp) is and how to prepare for the medical device process validation according to iso 13485. Learn.
Validation Master Plan Template Validation Center
Learn how to prepare a validation master plan (vmp) for drug product or active pharmaceutical ingredient manufacturing and. Buy a fully editable and compliant document template for recording the schedule and status of validations for medical devices. Three (3) options to create a validation master plan. You can create a great protocol, using a template. Discover the essential components of.
FREE 9+ Sample Validation Plan Templates in PDF MS Word
Discover the essential components of a validation master plan in the pharmaceutical and medical device industries, understanding its importance. Learn how to prepare a validation master plan (vmp) for drug product or active pharmaceutical ingredient manufacturing and. Buy a fully editable and compliant document template for recording the schedule and status of validations for medical devices. You can create a.
FREE 9+ Sample Validation Plan Templates in PDF MS Word
Learn what the validation master plan (vmp) is and how to prepare for the medical device process validation according to iso 13485. Three (3) options to create a validation master plan. Discover the essential components of a validation master plan in the pharmaceutical and medical device industries, understanding its importance. You can create a great protocol, using a template. Learn.
Validation Master Plan Egnyte
Learn what the validation master plan (vmp) is and how to prepare for the medical device process validation according to iso 13485. You can create a great protocol, using a template. Buy a fully editable and compliant document template for recording the schedule and status of validations for medical devices. Learn how to prepare a validation master plan (vmp) for.
Validation Master Plan. Understand the importance and benefits
You can create a great protocol, using a template. Learn how to prepare a validation master plan (vmp) for drug product or active pharmaceutical ingredient manufacturing and. Three (3) options to create a validation master plan. Learn what the validation master plan (vmp) is and how to prepare for the medical device process validation according to iso 13485. Buy a.
Three (3) options to create a validation master plan. Learn what the validation master plan (vmp) is and how to prepare for the medical device process validation according to iso 13485. You can create a great protocol, using a template. Learn how to prepare a validation master plan (vmp) for drug product or active pharmaceutical ingredient manufacturing and. Buy a fully editable and compliant document template for recording the schedule and status of validations for medical devices. Discover the essential components of a validation master plan in the pharmaceutical and medical device industries, understanding its importance.
Discover The Essential Components Of A Validation Master Plan In The Pharmaceutical And Medical Device Industries, Understanding Its Importance.
Three (3) options to create a validation master plan. Learn how to prepare a validation master plan (vmp) for drug product or active pharmaceutical ingredient manufacturing and. You can create a great protocol, using a template. Buy a fully editable and compliant document template for recording the schedule and status of validations for medical devices.